The Brett Helms Research Group, part of the California-based Lawrence Berkeley National Laboratory, has synthesised a new type of plastic polymer – poly(diketoenamine)s or PDKs – that can be completely recycled, even in the presence of additives like dyes, flame retardants and glass fibres. The discovery was published in Nature Chemistry in May 2019.
Between 2010 and today – according to data from the Centre for Environmental Law – major oil companies have invested over 180 billion dollars in the construction of new infrastructure for the production of plastics, over 99% of which are obtained from fossil fuels. A good way to make the plastic recycling process more economically advantageous and logistically simpler is to create products that are easily recyclable at a low cost. PDKs represent a new class of intelligent plastic polymers designed to be completely integrated into the circular economy.
PDKs are formed spontaneously at room temperature by mixing molecules of triketone and amines in a ball mill. This process leads to the formation of particular chemical bonds that require less energy to be broken than those present in traditional plastic polymers, which are difficult and costly to recycle. By varying the length of the mixing process – from a few minutes up to 45 – and the concentration of products PDKs with different and predictable properties can be created. Notably, PDKs can be created with the chemical and physical characteristics of typical thermosetting plastic polymers (rigidity, insolubility and infusibility) as well as of thermoplastic polymers (that can instead be remodelled at high temperatures).
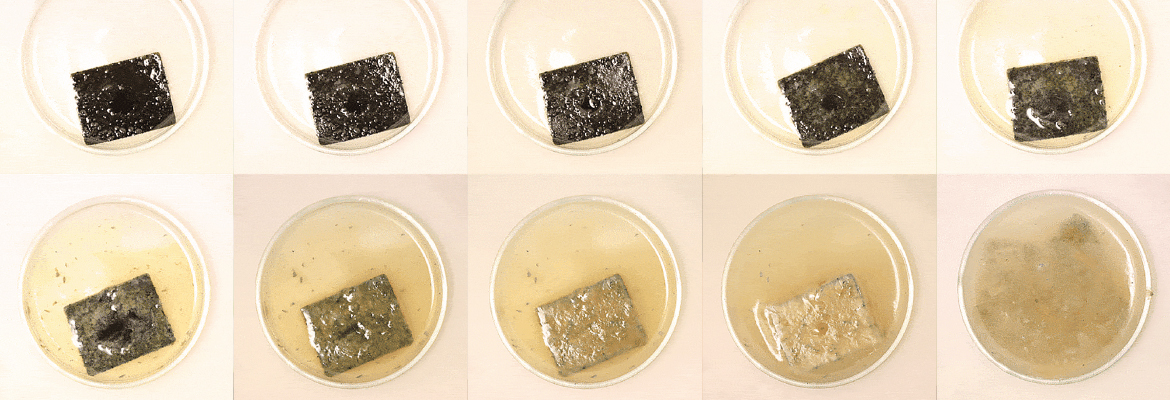
PDKs can be recycled through immersion in sulphuric or hydrochloric acid at room temperature, breaking down into their source monomers in only 12 hours.
A Polymer Made to be Recycled Easily
“The most important innovation was to show that all the additives that get compounded with plastics can be deconstructed and separated, leading to the recreation of the starting polymers – recycling – or adding value to the products – upcycling – by creating new polymers. At the end of the process the qualitative purity of the recovered source monomers is very high, without large quantitative losses or variations in their characteristics,” explains Brett Helms, Director of the Research Group.
To synthesise PDK, the researchers drew inspiration from existing recyclable thermosetting polymers and from so-called “click chemistry”, in which synthesis reactions use easily obtainable source materials, have high yields, only generate innocuous waste and create products that are easy to isolate.
“We asked ourselves how to obtain polymers that require smaller amounts of energy and water to be degraded compared with existing thermosetting polymers. We looked at what types of chemical bonds can degrade easily. The first test we attempted went well, and confirmed our hypothesis,” Helms recounts.
Separation From Dyes, Flame Retardants and Glass Fibres
The removal of additives entwined in plastics for mechanical or aesthetic reasons is one of the most complex and expensive parts of plastic recycling at an industrial level. “Being able to develop polymers that are completely recyclable and from which additives could also be removed, required several attempts and a certain amount of mistakes,” Helms admits. “We turned to our colleagues involved in the large-scale industrial production of plastic polymers, and asked them the types and amounts of dyes they use to make food packaging, or the amount of glass fibre used to strengthen plastic. We ran some experiments with additive quantities in accordance with industry standards, in an attempt to develop the circular economy of plastic in conditions similar to those present in the manufacturing industry.”
In an early experiment, the researchers added various dyes and carbon fibres to PDK. They were then recycled via immersion in a strong acid (like for pure PDKs), then in an aqueous solution from which the additives were separated through filtration. In a second experiment, flame retardants and glass fibres were added. In this case also, the separation process allowed for the recovery of the source molecules, as well as intact glass fibres and pure flame retardants, by adding only a single step compared to pure PDK recycling.
Recycling in the Presence of Other Plastic Polymers
In a third experiment, the researchers applied the process of PDK depolymerisation and recovery of source monomers in the presence of some of the most common types of plastic on the market today. Polymers such as polyethylene, nylon, polypropylene, polyvinyl chloride and polycarbonates do not react with strong acids at room temperature, and this allows to hypothesise that PDKs might even be recovered from undifferentiated mixed plastic waste.
Recycled PDK molecules from the various experiments were then used to re-synthesise the same type of PDK, or to obtain different polymers: the resulting products are in no way different to those made from PDKs synthesised from virgin raw materials.
The Transition From Academic Research to Industrial Production
In developing PDKs, the researchers took into account not only aspects of industrial production, but also those related to placement on the market. “We chose to produce PDKs via a dry method to avoid the use of unstable organic compounds, which are the ones that give that new car smell,” explains Helms. “Some people like this smell, others do not. Furthermore, these products are subject to regulations, and in some Asian countries, China especially, are mistrusted and their presence would compromise the products’ market appeal.”
Another important aspect is cost, as well as the possibility of resupply for source materials. “PDKs are made from the same constituent elements as nylon and polyurethane, two common polymers that on an industrial level have a cost that varies from 3,000 to 15,000 dollars a tonne, and that’s without the need to create a supply chain,” says Helms. “For production at an industrial level – the researcher continues – it will be necessary to study how to optimise the quantity of strong acid used for recycling. Strong acids are not commonly used in the plastics industry, but they are easy to obtain at a low price.”
In the plastics recycling industry the grinding of waste is a consolidated practice, however this is damaging to certain types of plastic, such as polyethylene, for example. “We cannot think that we will change the practice of grinding waste,” states Helms. “Therefore the question is: to what point does grinding damage PDKs, and what can we do to diminish this impact?”
The Search For Products That Can Be Integrated in the Circular Economy
“Every time a new type of plastic is created we try to understand what the best products that it can be used for are. Now we are trying to develop specific formulations for PDKs for specialised applications, so as to create products that are completely integrated in the circular economy,” Helms adds.
His research group is now working on elastomers, a category of soft and flexible plastics that have the chemical and physical characteristics typical of natural rubber and are used for footwear and other purposes where objects need to change shape as they are used, but return to their original shape and size when they are at rest.
Lawrence Berkeley National Laboratory, https://www.lbl.gov
Helms Group, https://foundry.lbl.gov/helmsgroup
AAVV, “Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds”, Nature Chemistry, 2019, v. 11, p. 442-448; www.nature.com/articles/s41557-019-0249-2
Top image: Unlike conventional plastics, the monomers of PDK plastic could be recovered and freed from any compounded additives simply by dunking the material in a highly acidic solution. Peter Christensen et al./Berkeley Lab